What Is the Mass of an Aspirin Tablet
250 milligrams equals 025 grams so the mass is 025 grams. What is the molar mass of aspirin.

Solved 2 Ifyour Aspirin Tablet Contains 325mg Of Asa Acetylsalicylic Acid Theoretically Quesdon Calculate Mass Of Excipient Based On The Weight Of Your Aspirin Tablet 10 Rts Mass Of One Tablet Mass Of
Aspirin also known as acetylsalicylic acid ASA is a medication used to reduce pain fever or inflammation.

. Up to 25 cash back Data. The molar mass of aspirin 2-acetoxybenzoic acid C9H8O4 is 180157 gmol. An aspirin tablet contains 325 mg of acetylsalicylic acid.
So aspirin C9H8O4 has a molar mass of 180157 g mol1. 1000g 1 mole aspirin 180157g 055507 moles aspirin. 300 to 600 mg rectally every 4 hours.
Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease pericarditis and rheumatic fever. Melting range 1282- read more David the Scientist. Calculate the total mass of aspirin in a 500-tablet bottle in grams and ounces.
The mass of oxalic acid H2C2O4aq and Equation 4 are then used to determine the number of moles of potassium hydroxide present. Assume that it takes 2375mL of a 007980 M NaOH to reach the end point. So there is 325180 18 mol.
Most OTC drugs are not reviewed and approved by FDA however they may be marketed if they comply with applicable regulations and policies. Molar mass of C9H8O4 18015742 gmol. Aspirin given shortly after a heart attack decreases the risk of death.
Computed by PubChem 21 PubChem release 20210507 Monoisotopic. 1615 mL of base solution is required to neutralize A Bayer aspirin tablet the mass of which. The point here is to see how much aspirin you found.
You will first standardize your NaOH solution and then use it to analyze aspirin tablets for their aspirin content and purity. An aspirin tablet may also include inactive ingredients that help produce a consistent product for consumers. Think of the purpose these ingredients serve when considering whether your percent purity is.
An aspirin tablet may also include inactive ingredients that help produce a consistent product for consumers. 37 Learn how to calculate the average mass of aspirin in tablets using a back titration 38 How To Calculate The Molar Mass of a Compound Quick Easy. Convert grams Aspirin.
And the gram formula mass of the molecule is 180 gmol. 300 to 650 mg orally every 4 to 6 hours as needed. This means that one mole of aspirin will have a mass of 180157 g.
You are starting with the mass of the tablet. You take an aspirin tablet a compound consisting solely of carbon hydrogen and oxygen with a mass of 100 g combust it under an oxygen atmosphere and collect 220 g of carbon dioxide and 0400 g water. Aspirin is also used long-term to help prevent further heart attacks ischaemic.
2 g is approx 1100 of a mole. 8 rows Exact Mass. 1525 mL of base solution is required to neutralize 0312 g of pure aspirin.
View the full answer. 2 The following data are collected in an experiment to determine the amount of aspirin the compound in a Bayer aspirin tablet. 36 Calculate the mass percentage of aspirin C9H8O4 in acetonitrile CH3CN when 65g of C9H8O4.
C9H8O4 in one aspirin tablet that weighs 03752g. A What is the mass of acetylsalicylic acid Formula. Determining the precise concentration of NaOH using a primary standard is called standardization.
Now that you know how many moles of aspirin you have in your sample use the fact. The calculation is wrong. 4 rows Aspirin molecular weight.
As a temporary fever reducer or for the temporary relief of minor pain due to headache menstrual pain arthritis muscle pain or. What is the mass of acetylsalicylic acid in the aspirin tablet. That should be obvious.
Mass 02063g ViNaOH 790 ml Vf NaOH 1290ml V used NaOH 500ml Concentration of NaOH 02500m obs. An aspirin tablet has a mass of about 325mg. 4 g in 24 hours.
Use the M you calculated. Answer Expert Verified The formula of acetylsalicylic acid is C9H8O4. MEDIQUE ASPIRIN- aspirin tablet film coated MEDI-FIRST ASPIRIN- aspirin tablet film coated MEDI FIRST PLUS ASPIRIN- aspirin tablet film coated Unifirst First Aid Corporation Disclaimer.
Youre dealing with a 1000-g sample of aspirin which will be equivalent to. What is the mass in grams of a 250 mg aspirin tablet. You dont want that.
Assume that it takes 2375mL of a 007980 M NaOH to reach the end point. Moles of aspirin 20729g180154gmol-1 01154 mol. Subsequently question is how many grams of acetylsalicylic acid are in the tablet.
How much aspirin that. B 330 mg 2.

Titration Of Aspirin Lab Macro Lab Student Data Cooperative Learning Chemistry Class

Learn How To Calculate The Average Mass Of Aspirin In Tablets Using A Back Titration Youtube
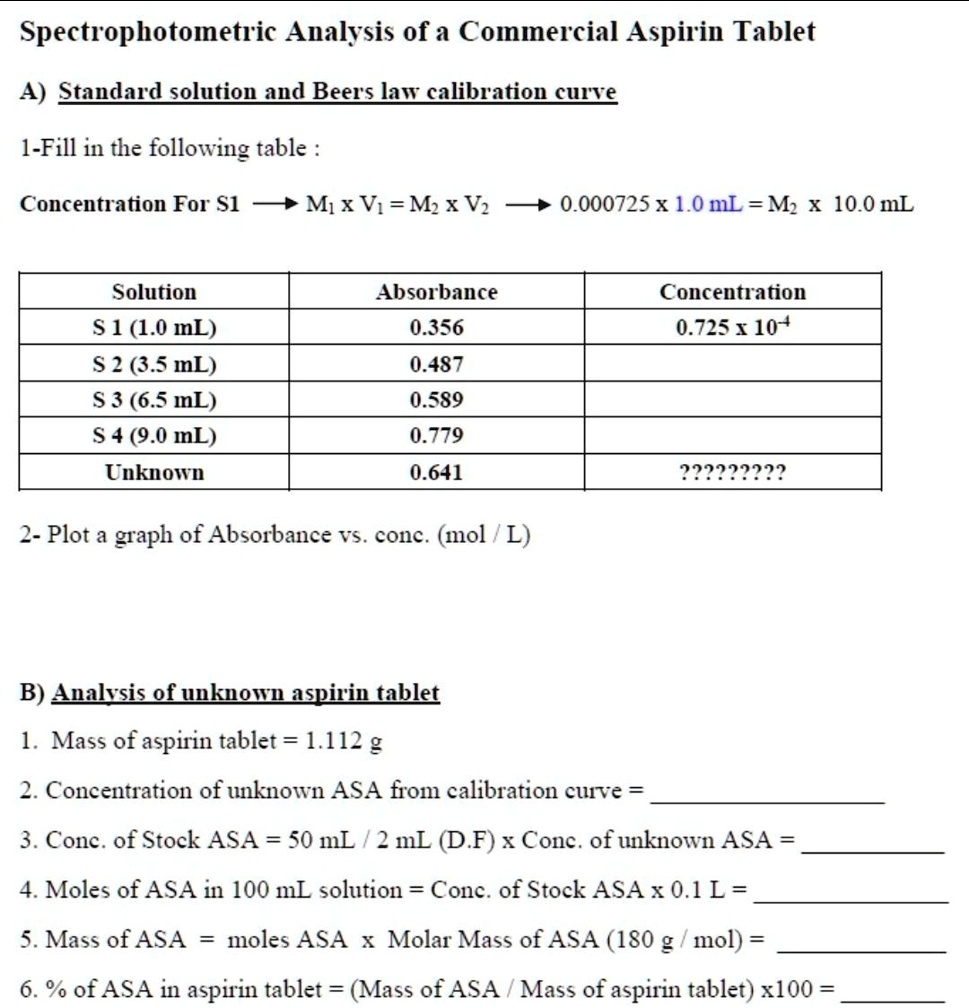
Solved Spectrophotometric Analysis Of A Commercial Aspirin Tablet A Standard Solution Aud Beers Law Calibration Cure 1 Fill In The Following Table Concentration For Sl Mx Vi Mx Vz 0 000725x 1 0 Ml M 10 0
Comments
Post a Comment